Multiple myeloma (MM) is a genetically complex hematological disease which is characterized by clonal proliferation of plasma cells in the bone marrow and secretion of monoclonal antibodies and cytokines that can damage bone, bone marrow, and kidney function1. MM cells constantly operate at the limit of their unfolded protein response (UPR) in the face of a secretory load of immunoglobin (Ig) and cytokines that is unparalleled by any other mammalian cell 2,3 and microenvironmental factors that aggravate the degree of physiologic misfolding that occurs during synthesis of secreted proteins. The endoplasmic reticulum (ER) resident protein disulfide isomerases (PDIs) are indispensable for folding of secreted proteins that require intramolecular disulfide-bond arrangement 4 like antibodies and many cytokines. As the main PDI family member, near-complete function of PDIA1 is essential for survival of MM cells while its inhibition should be manageable by the UPR in normal cells creating an opportunity for a large therapeutic window for PDI inhibitors in MM.
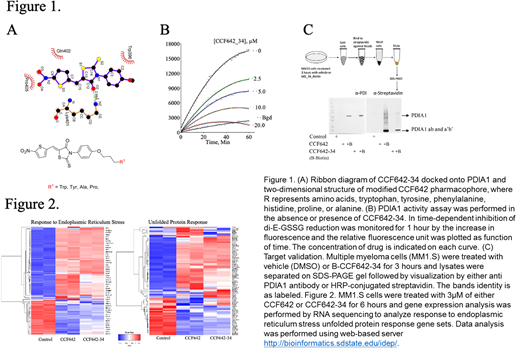
Previously, we discovered and characterized an irreversible PDI inhibitor (CCF642) that induced cell death in MM cells at doses that did not affect survival of normal bone marrow cells. However, CCF642 has poor solubility and suboptimal selectivity precluding clinical translation. Using structure guided medicinal chemistry, we developed and characterized a highly potent and selective PDI inhibitor, with 10-fold higher potency (Fig 1B) and selectivity. CCF642-34 showed remarkable selectivity against PDIA1 and off-target bindings were eliminated when compared to CCF642 (Fig 1C). In addition to improved selectivity and in vitro PDI inhibition, CCF642-34 demonstrated more than 3-fold higher potency compared to CCF642 against MM1.S and bortezomib resistant MM1.S cells remained sensitive to CCF642-34. Importantly, the novel analogue CCF642-34 has 18-fold better potency in restricting the colony forming abilities of RPMI1640 cells while at no effect on the clonogenic potential of CD34+ cells derived from healthy bone marrow was observed at equivalent doses. CCF642-34 induces ER stress in MM1.S cells as observed in dose and time dependent cleavage of XBP1, IRE1α oligomerization and the profound induction of programmed cell death reflected by PARP and caspase 3 cleavage.
To further analyze the modes of action of CCF642-34 and CCF642 we performed RNAseq after treatment of MM1.S cells and found exclusive induction of genes associated with UPR and downstream cell cycle and apoptotic responses for CCF642-34 while additional genes affecting were detected after CCF642 treatment. There were 362 and 568 differentially expressed genes in CCF642-34 and CCF-642 (compared to controls) treated MM1.S cells, respectively. Among these differentially expressed genes 87 down regulated and 142 upregulated were common to both, including downregulation of cell division and mitotic cell cycle process, and upregulation of response to ER stress, unfolded protein response, and apoptotic process gene sets. Results confirm that both CCF642 and CCF642-34 treatment act by inducing lethal ER-stress with greater selectivity for CCF642-34. Accordingly, hierarchical clustering showed distinct gene expression profiles in 642-34 and 642 treated MM1S cells (Fig. 2).
CCF642-34 is orally bioavailable and highly efficacious in against established multiple myeloma in a syngeneic 5TGM1-luc/C57BL/KaLwRij model of myeloma. All vehicle control animals were dead by 52 days while 3 out of 6 mice lived beyond 6 months with no sign of relapse. In summary, we synthesized and characterized a novel lead PDIA1 inhibitor based on structure-guided medicinal chemistry that has improved pharmacologic properties to act as novel lead for clinical translation.
References:
1. Manier S, Salem KZ, Park J, et al. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017;
2. Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: Spotlight review. Leukemia. 2009;
3. Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014;
4. Freedman RB, Hirst TR, Tuite MF. Protein disulphide isomerase: building bridges in protein folding. Trends Biochem. Sci. 1994;
Valent:Takeda Pharmaceuticals: Other: Teaching, Speakers Bureau; Celgene: Other: Teaching, Speakers Bureau; Amgen Inc.: Other: Teaching, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.